Medsafe replicates in part or in whole the drug approval processes of its overseas counterparts. There is expedited processing for drugs already approved overseas.
Every day in which a drug approval application is sitting on the desk of a bureaucratic at Medsafe is a day in which another New Zealander may die but for that drug.
That delay in access to drugs because of the duplication in approval processes is the invisible graveyard of Medsafe. My Official Information Act requests so far have been unable to access a cost benefit analysis at the Ministry of Health that quantifies the size of that invisible graveyard.

If economists have a bitter drinking song it would be “how many people has the FDA killed today”. Many drugs became available years after they were on the market outside the USA because of drug approval lags at the FDA. The dead are many. To quote David Friedman:
In 1981… the FDA published a press release confessing to mass murder. That was not, of course, the way in which the release was worded; it was simply an announcement that the FDA had approved the use of timolol, a ß-blocker, to prevent recurrences of heart attacks. At the time timolol was approved, ß-blockers had been widely used outside the U.S. for over ten years.
It was estimated that the use of timolol would save from seven thousand to ten thousand lives a year in the U.S. So the FDA, by forbidding the use of ß-blockers before 1981, was responsible for something close to a hundred thousand unnecessary deaths.
The only rational basis for duplicating overseas drug safety approval processes is the honest belief that a New Zealand process can pick up errors. These errors must be so large that they justify the delay. If there are no such errors to pick up in a cost justified manner, the drug approval branch of Medsafe just adds to the invisible graveyard.
The Ministry of Health did advise its Minister of the unilateral recognition model in Singapore. If a drug is registered in two trusted jurisdictions, it is fast tracked. If it is registered in one other trusted jurisdiction, it goes through an expedited process. This process appears to only cut the registration process for a drug from 270 days to 240 days.
The truncated approaches when there is approval of the drug in a trusted jurisdiction usually call for access to evaluation reports and other red tape. What can drug trials in New Zealand
find out that is not already known? Medsafe targets processing applications for the approval of new drugs in New Zealand to be done within 200 days. That’s 200 days too many.

My preferred unilateral recognition process consists of authenticating the drug registration certificate from a trusted overseas jurisdiction. It would be a post-box process. The G7 countries plus Australia should be this list of trusted overseas jurisdiction. There should be automatic recognition in New Zealand of any drug registration in those jurisdictions.
There is no reason to believe that Medsafe will pick up errors of trusted jurisdictions overseas. Medsafe denied New Zealanders access to four drugs approved in comparable regulatory jurisdictions in the last three years. Medsafe rejected two other drugs in the last three years but these drugs were not approved in comparable jurisdictions. Medsafe is not involved in the funding of medicines; this is the responsibility of PHARMAC.
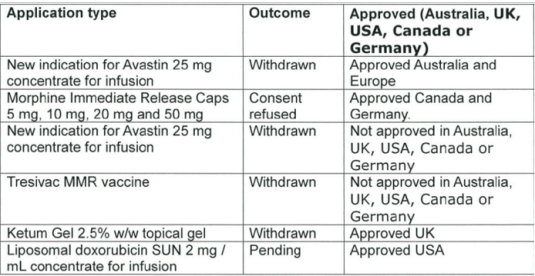
Source: data released 29 October 2015 pursuant to an Official Information Act request to the Ministry of Health.
Medsafe is turning down not even a handful of drugs were approved overseas jurisdictions in the past three years. Was that worth the wait? Was that worth a larger invisible graveyard?
The net benefits of the entire drug approval framework over the past three years in New Zealand is riding out on rejecting for approval half a dozen drugs, four of which are approved as safe in other comparable jurisdictions.
The size of the invisible graveyard has been quantified in the USA. The Prescription Drug User Fee Acts (PDUFA) reduced the drug approval time lag by 10 months:
Converting these economic gains into equivalent health benefits, we find that the more rapid access of drugs on the market enabled by PDUFA saved the equivalent of 140,000 to 310,000 life years.
A few drugs were approved that were later withdrawn. Their unfortunate consequences must always be weighed against the drugs that got to the market faster, saving lives, relieving pain and curing illnesses. That trade-off must be faced up to openly rather than as it is now left in the invisible graveyard.

Dec 23, 2015 @ 11:21:16
Reblogged this on Utopia – you are standing in it! and commented:
LikeLike