
From http://www.nzlii.org/nz/legis/hist_act/qpa19088ev1908n247335/
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
03 Feb 2021 Leave a comment
in economics of regulation, health economics, politics - New Zealand Tags: Drug safety
19 Nov 2020 Leave a comment
in gender, health economics Tags: Drug safety


13 Mar 2018 Leave a comment
in applied price theory, applied welfare economics, economics of information, economics of regulation, environmental economics, health economics Tags: drug lags, Drug safety, Product safety, The fatal conceit
02 Feb 2017 Leave a comment
in applied price theory, economic history, economics of regulation, health economics Tags: drug lags, Drug safety
17 Dec 2015 1 Comment
in economics of bureaucracy, economics of regulation, health economics, politics - New Zealand Tags: drug lags, Drug safety
Medsafe replicates in part or in whole the drug approval processes of its overseas counterparts. There is expedited processing for drugs already approved overseas.
Every day in which a drug approval application is sitting on the desk of a bureaucratic at Medsafe is a day in which another New Zealander may die but for that drug.
That delay in access to drugs because of the duplication in approval processes is the invisible graveyard of Medsafe. My Official Information Act requests so far have been unable to access a cost benefit analysis at the Ministry of Health that quantifies the size of that invisible graveyard.

If economists have a bitter drinking song it would be “how many people has the FDA killed today”. Many drugs became available years after they were on the market outside the USA because of drug approval lags at the FDA. The dead are many. To quote David Friedman:
In 1981… the FDA published a press release confessing to mass murder. That was not, of course, the way in which the release was worded; it was simply an announcement that the FDA had approved the use of timolol, a ß-blocker, to prevent recurrences of heart attacks. At the time timolol was approved, ß-blockers had been widely used outside the U.S. for over ten years.
It was estimated that the use of timolol would save from seven thousand to ten thousand lives a year in the U.S. So the FDA, by forbidding the use of ß-blockers before 1981, was responsible for something close to a hundred thousand unnecessary deaths.
The only rational basis for duplicating overseas drug safety approval processes is the honest belief that a New Zealand process can pick up errors. These errors must be so large that they justify the delay. If there are no such errors to pick up in a cost justified manner, the drug approval branch of Medsafe just adds to the invisible graveyard.
The Ministry of Health did advise its Minister of the unilateral recognition model in Singapore. If a drug is registered in two trusted jurisdictions, it is fast tracked. If it is registered in one other trusted jurisdiction, it goes through an expedited process. This process appears to only cut the registration process for a drug from 270 days to 240 days.
The truncated approaches when there is approval of the drug in a trusted jurisdiction usually call for access to evaluation reports and other red tape. What can drug trials in New Zealand
find out that is not already known? Medsafe targets processing applications for the approval of new drugs in New Zealand to be done within 200 days. That’s 200 days too many.
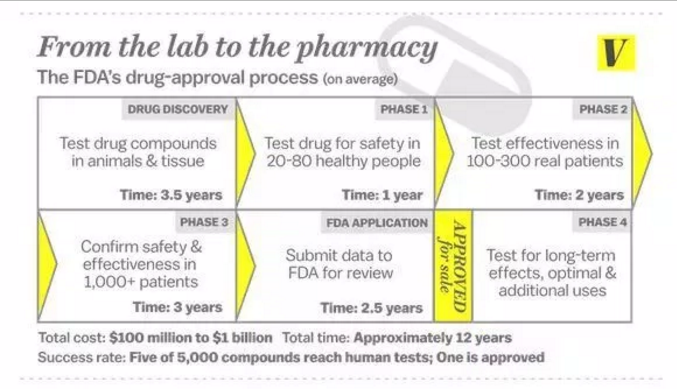
My preferred unilateral recognition process consists of authenticating the drug registration certificate from a trusted overseas jurisdiction. It would be a post-box process. The G7 countries plus Australia should be this list of trusted overseas jurisdiction. There should be automatic recognition in New Zealand of any drug registration in those jurisdictions.
There is no reason to believe that Medsafe will pick up errors of trusted jurisdictions overseas. Medsafe denied New Zealanders access to four drugs approved in comparable regulatory jurisdictions in the last three years. Medsafe rejected two other drugs in the last three years but these drugs were not approved in comparable jurisdictions. Medsafe is not involved in the funding of medicines; this is the responsibility of PHARMAC.
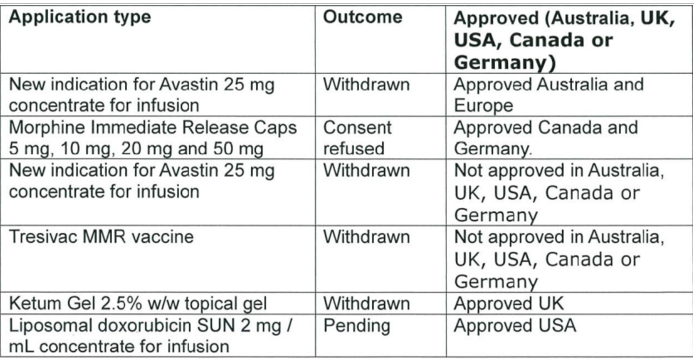
Source: data released 29 October 2015 pursuant to an Official Information Act request to the Ministry of Health.
Medsafe is turning down not even a handful of drugs were approved overseas jurisdictions in the past three years. Was that worth the wait? Was that worth a larger invisible graveyard?
The net benefits of the entire drug approval framework over the past three years in New Zealand is riding out on rejecting for approval half a dozen drugs, four of which are approved as safe in other comparable jurisdictions.
The size of the invisible graveyard has been quantified in the USA. The Prescription Drug User Fee Acts (PDUFA) reduced the drug approval time lag by 10 months:
Converting these economic gains into equivalent health benefits, we find that the more rapid access of drugs on the market enabled by PDUFA saved the equivalent of 140,000 to 310,000 life years.
A few drugs were approved that were later withdrawn. Their unfortunate consequences must always be weighed against the drugs that got to the market faster, saving lives, relieving pain and curing illnesses. That trade-off must be faced up to openly rather than as it is now left in the invisible graveyard.
29 Oct 2015 1 Comment
in applied welfare economics, economics of regulation, health economics, politics - New Zealand Tags: drug lags, Drug safety
Medsafe denied New Zealanders access to four drugs approved in comparable regulatory jurisdictions in the last three years. Medsafe rejected two other drugs in the last three years but these drugs were not approved in comparable jurisdictions. Doxorubicin Liposomal, chemotherapy drug, is not as yet actually refused, its application is pending. Medsafe is not involved in the funding of medicines; this is the responsibility of PHARMAC.
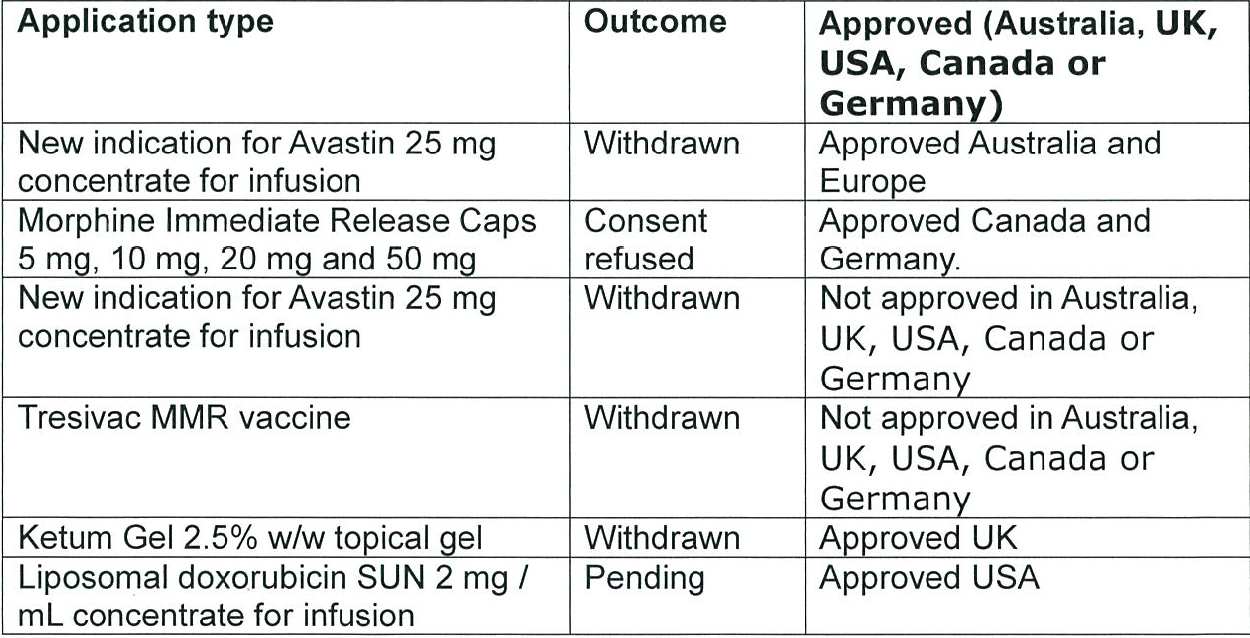
Source: data released 29 October 2015 pursuant to an Official Information Act request to the Ministry of Health.
What’s the point of this regulatory arm of the Ministry of Health? Is it a waste of space? Should not New Zealand automatically register any drug approved in the USA, UK, Canada, Australia or Germany? What can medical trials in New Zealand find out were not already found out overseas? Medsafe targets processing applications for the approval of new drugs in New Zealand to be done within 200 days. That’s 200 days too many.
Barriers to entry – costs ($s) of bringing new drugs to the market have risen manyfold #econ3 http://t.co/MFHTFhTLrj—
Geoff Riley (@tutor2u_econ) November 30, 2014
It should be lawful under the Medicines Act 1981 to market any drug in New Zealand which any of Australia, UK, USA, Canada or Germany has approved for prescription to patients.
SEEN & UNSEEN/ How much illness & death could be averted by limiting the FDA to safety, leaving efficacy to markets? http://t.co/4QkUuCCMDN—
Robert Graboyes (@Robert_Graboyes) March 11, 2015
If economists have a bitter drinking song, a battle cry that unites the warring schools of economic thought all, it would be “how many people has the FDA killed today”. For example, drugs became available years after they were on the market outside the USA because of drug approval lags at the FDA. The dead are many. To quote David Friedman:
In 1981… the FDA published a press release confessing to mass murder. That was not, of course, the way in which the release was worded; it was simply an announcement that the FDA had approved the use of timolol, a ß-blocker, to prevent recurrences of heart attacks. At the time timolol was approved, ß-blockers had been widely used outside the U.S. for over ten years. It was estimated that the use of timolol would save from seven thousand to ten thousand lives a year in the U.S. So the FDA, by forbidding the use of ß-blockers before 1981, was responsible for something close to a hundred thousand unnecessary deaths.
In 1962, an amended law gave the FDA authority to judge if a new drug produced the results for which it had been developed. Formerly, the FDA monitored only drug safety. It previously had only sixty days to decide this. Drug trials can now take up to 10 years.
Sam Peltzman showed in a famous paper in 1973 that these 1962 amendments reduced the introduction of new drugs in the USA from an average of forty-three annually in the decade before the 1962 amendments to sixteen annually in the ten years afterwards. No increase in drug safety was identified.
Halving every 9 years.
New approved drugs per billion dollars spent on research & development
bit.ly/1StxuV9 http://t.co/Hvx6mQ0lBa—
Max Roser (@MaxCRoser) August 01, 2015
Medsafe is a cost with no benefits to the New Zealand public. Medsafe has around 60 staff operating out of two offices, with centralised administrative functions, product approval and standard setting at the head office in Wellington.
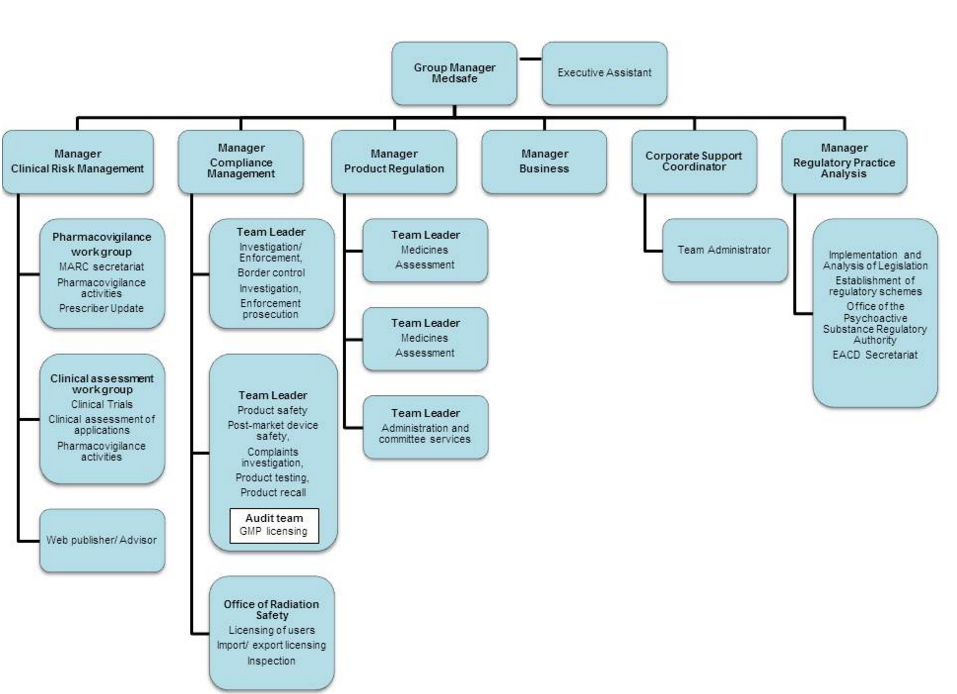
How much of this budget of several million for Medsafe could be redirected to funding more life-saving and life changing drugs for use in New Zealand? This is rather than wasted on duplicating clinical trials already completed overseas or at the minimum duplicating regulatory approval processes, paperwork already completed overseas but not requiring a duplicate clinical trial in New Zealand.
At a minimum, the net benefits of the entire drug approval framework over the past three years in New Zealand is riding out on rejecting for approval half a dozen drugs, four of which are approved as safe in other comparable jurisdictions. That’s a pretty thin reed on which to hang a large budget that could be used by PHARMAC to fund life-saving drugs.
There should be a post box at the Ministry of Health to receive the certifications from overseas drug regulation agencies. Anything more is a deadly waste of taxpayers’ money.
My next round of Official Information Act requests will ask whether the minister and associate ministers of health were briefed on refusals of new medicines approved in other jurisdictions. Next I will ask:
I have previously asked for information on drug approval lags. That was refused on the grounds I can look it up for myself on a rather complicated public database that requires knowledge of the names of medicines submitted for approval. Still mulling over what to do about that.
29 Mar 2015 Leave a comment
in economics of crime, economics of media and culture, law and economics Tags: baby boomers, drug abuse, Drug safety, The 60s
Chart: Baby Boomers got high way more than Millennials wapo.st/1GjUP4N http://t.co/VYQR1Q5Iq4—
Know More (@knowmorewp) March 04, 2015

14 Mar 2015 Leave a comment
in economics of regulation, Sam Peltzman Tags: drug lags, Drug safety
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Scholarly commentary on law, economics, and more
Beatrice Cherrier's blog
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Why Evolution is True is a blog written by Jerry Coyne, centered on evolution and biology but also dealing with diverse topics like politics, culture, and cats.
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
A rural perspective with a blue tint by Ele Ludemann
DPF's Kiwiblog - Fomenting Happy Mischief since 2003
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
The world's most viewed site on global warming and climate change
Tim Harding's writings on rationality, informal logic and skepticism
A window into Doc Freiberger's library
Let's examine hard decisions!
Commentary on monetary policy in the spirit of R. G. Hawtrey
Thoughts on public policy and the media
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Politics and the economy
A blog (primarily) on Canadian and Commonwealth political history and institutions
Reading between the lines, and underneath the hype.
Economics, and such stuff as dreams are made on
"The British constitution has always been puzzling, and always will be." --Queen Elizabeth II
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
WORLD WAR II, MUSIC, HISTORY, HOLOCAUST
Undisciplined scholar, recovering academic
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Res ipsa loquitur - The thing itself speaks
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Researching the House of Commons, 1832-1868
Articles and research from the History of Parliament Trust
Reflections on books and art
Posts on the History of Law, Crime, and Justice
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Exploring the Monarchs of Europe
Cutting edge science you can dice with
Small Steps Toward A Much Better World
“We do not believe any group of men adequate enough or wise enough to operate without scrutiny or without criticism. We know that the only way to avoid error is to detect it, that the only way to detect it is to be free to inquire. We know that in secrecy error undetected will flourish and subvert”. - J Robert Oppenheimer.
The truth about the great wind power fraud - we're not here to debate the wind industry, we're here to destroy it.
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Economics, public policy, monetary policy, financial regulation, with a New Zealand perspective
Celebrating humanity's flourishing through the spread of capitalism and the rule of law
Restraining Government in America and Around the World
Recent Comments